Appending cation onto manganese imido complex affects the strength of N—H bond
Teera Chantarojsiri
Ammonia (NH3) is a key molecule in the nitrogen cycle and can potentially be chemical fuel due to its high energy density. In order to use ammonia as fuel, metal complex can be used as catalysts, generating metal-imido complex as an intermediate. In this work, manganese imido complex with appended cation were synthesized and studied. The electrostatic effects, generated by the proximal cation, altered the redox potential, pKa, and bond dissociation free energy.
This work is done in a collaboration with Professor Jenny Y. Yang from the Department of Chemistry, University of California, Irvine. Knowledge obtained from this work will benefit researchers who are working on modulating bond dissociation energy of different molecules that can be candidates for chemical fuels. Associated SDG goals are Affordable and Clean Energy (7) and Partnerships for the Goals (17).
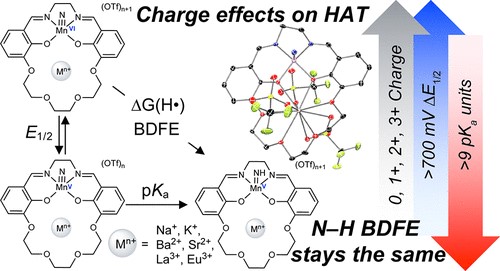
Reference: Cationic Effects on the Net Hydrogen Atom Bond Dissociation Free Energy of High-Valent Manganese Imido Complexes
Léonard, N.G., Chantarojsiri, T., Ziller, J.W., Yang, J.Y.
Journal of the American Chemical Society, 2022, 144(4), pp. 1503–1508
