Muhammad Dzulfahmi Ramadhan† and Panida Surawatanawong*,†,‡
†Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
‡Center of Sustainable Energy and Green Materials, Mahidol University, Salaya, Nakhon Pathom 73170, Thailand
*E–mail: panida.sur@mahidol.ac.th
Understanding CO2 addition and H2 activation by geminal P/Z (Z = B, Al) frustrated Lewis pairs (FLPs) is crucial for the development of transition metal-free catalysts for hydrogenation of unsaturated compounds and CO2 conversion. The distortion–interaction energy decomposition and natural bond orbital were used to gain insights into the reactivity of CO2 addition and H2 activation. Remarkably, the orbital interactions in the reactions of CO2 and H2 with geminal P/B FLPs are stronger than those in the reactions with more reactive geminal P/Al FLPs, which contain a stronger Lewis acid. The fact that geminal P/B FLPs react with a higher energy barrier than geminal P/Al FLPs is actually due to the unfavorable core repulsion contribution to the interaction energy and the higher distortion energy found for the geminal P/B FLPs. In addition, we revealed that although the interaction energy is higher for H2 activation, the distortion energy is significantly lower for CO2 addition. Thus, geminal P/Z FLPs are more reactive toward CO2 than toward H2. Overall, the reactivity and thermodynamic stability for the reactions of geminal P/Z FLPs with CO2 and H2 are not affected by the type of bridging carbon (sp3-C or sp2-C) but mainly influenced by the substituents on P and Z (B or Al). An insight into the thermodynamic stability of the H2 activation product is gained by quantifying the hydride affinity, the proton affinity, and the stability of the zwitterionic product relative to the prototypical Lewis acid–base pair, PtBu3/B(C6F5)3.
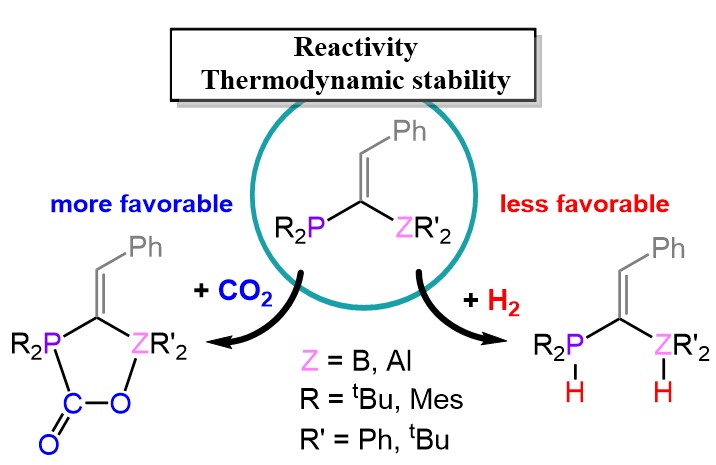
Reference: Ramadhan, M. D.; Surawatanawong*, P. Dalton Trans., 2021, 50, 11307–11316. https://pubs.rsc.org/en/content/articlelanding/2021/dt/d1dt01535d
