Exogenous addition was used to conduct a model study on the effect of certain heavy metal ions on the stability and vulcanization efficiency of uncompounded and compounded high-ammonia natural rubber (HANR) latex, which was then measured using a Brookfield viscometer, mechanical stability time (MST) tester, and tensile testing machine. The case of pre-vulcanized HANR latex with varying age durations was assessed by the change in the number of volatile fatty acids (VFA), MST, and viscosity. Mn2+ and Mg2+ ions coagulated the compounded HANR latex, but Zn2+, Fe2+, and Cu2+ ions had no effect, resulting in colloidal stability. Consequently, these metal ions were considered for future research into the pre-vulcanization of compounded HANR latex. The presence of Zn2+, Fe2+, and Cu2+ in latex causes the vulcanization process to be delayed and impacts the appearance of compounded latex. Before compounding, the gloves’ tensile strength was diminished by incorporating such metal ions. In addition, the tensile qualities of the gloves manufactured from the compounded HANR latex-containing metal ions were unaffected.
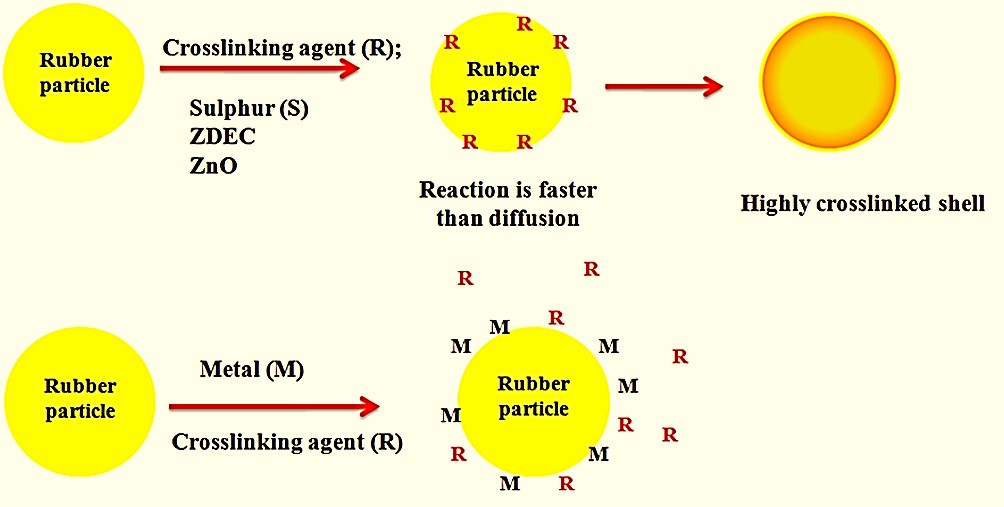
Reference: Rojruthai P, Payungwong N, Sakdapipanich J. A Model Study on the Impact of Metal Ions on Prevulcanization of Concentrated Natural Rubber Latex and Dipped-Products. Progress in Rubber Plastics and Recycling Technology. 2022;38(1):125-138.
