Bioinspired stereoselective synthesis of chiral 2,5-diaryl-3,4-dimethyltetrahydrofurans from unprotected 1,4-diarylbutane-1,4-diols
Rungrawin Chatpreecha, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul and Darunee Soorukram*
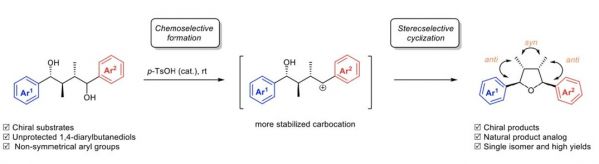
A bioinspired stereoselective synthesis of (2,3-anti-3,4-syn-4,5-anti)-2,5-diaryl-3,4-dimethyltetrahydrofurans from unprotected chiral 1,4-diarylbutane-1,4-diols is described. Upon treatment of the chiral 1,4-diarylbutane1,4-diols with acid, chiral 2,5-diaryl-3,4-dimethyltetrahydrofurans were obtained in high yields and high stereoselectivity through the chemoselective formation of a more stabilized benzylic carbocation followed by a stereoselective cyclization. Proposedly, the carbocation formation was chemoselectively governed by the substitution patterns of the non-symmetrical aryl groups of the 1,4-diarylbutane-1,4-diols and the stereoselective cyclization of the carbocation was inherently controlled by the stereochemistry of the substrates. The present study highlights a practical and an atom-economic process and provides essential information applicable for further design of the asymmetric synthesis of naturally occurring 2,5-diaryl-3,4- dimethyltetrahydrofurans and their derivatives isolated from Krameria cystisoides.
Reference: Bioinspired stereoselective synthesis of chiral 2,5-diaryl-3,4-dimethyltetrahydrofurans from unprotected 1,4-diarylbutane-1,4-diols. Rungrawin Chatpreecha, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul and Darunee Soorukram*. Arkivoc 2020, 6, 299-311. https://doi.org/10.24820/ark.5550190.p011.276
