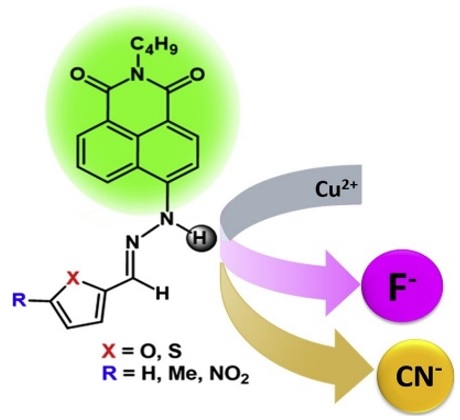
Four dual optical signalings of fluorescent hydrazone Schiff bases were synthesized from 1,8-naphthalimide hydrazide and substituted furan and thiophene rings, employing ethanol as green solvent. All synthesized molecules can detect F− and CN− with fast response with naked eye color change and quenching of fluorescence. Most common competitive anions have paltry interferences during the optical sensing of F−, while only nitro and methyl furan substituted provided good selectivity to CN− in THF. Substituents on heterocyclic directly affect fluoride capturing sensitivity, namely, electron donating group provides more sensitivity than with electron withdrawing groups. 1H NMR confirms the H-bonding between sensor molecule and F−/CN−. The detection limits of the four-sensor molecules were found below 0.3 ppm for F− and CN− detection. The magnitude of fluorescence quenching was estimated through Stern-Volmer plots. Test strips experimentation revealed the on-site solid-state detection efficacy of the sensors. Addition of Cu(II) ions to nitro and methyl furan substituted, resulted in selective discrimination between F− and CN− in THF. Computational studies prove the agreement of reactivity for four optical molecules interaction with F− and show that substituent at furan/thiophene does not affect the sensitivity, this is contrary to traditional school of thoughts.
Reference
“Furan/Thiophene-Based Fluorescent Hydrazones as Fluoride and Cyanide Sensors” Saini, N.; Wannasiri, C.; Chanmungkalakul, S.; Prigyai, N.; Ervithayasuporn, V.*; Kiatkamjornwong, S.* J. Photochem. Photobiol., A. 385 (2019) 112038. https://www.sciencedirect.com/science/article/pii/S1010603019310391
