Kaipeng Hou, Jonas Börgel, Henry Z. H. Jiang, Daniel J. SantaLucia, Hyunchul Kwon, Hao Zhuang, Khetpakorn Chakarawet, Rachel C. Rohde, Jordan W. Taylor, Chaochao Dun, Maria V. Paley, Ari B. Turkiewicz, Jesse G. Park, Haiyan Mao, Ziting Zhu, E. Ercan Alp, Jiyong, Michael Y. Hu, Barbara Lavina, Sergey Peredkov, Xudong Lv, Julia Oktawiec, Katie R. Meihaus, Dimitrios A. Pantazis, Marco Vandone, Valentina Colombo, Eckhard Bill, Jeffrey J. Urban, R. David Britt, Fernande Grandjean, Gary J. Long, Serena DeBeer, Frank Neese, Jeffrey A. Reimer, Jeffrey R. Long
Nature uses oxygen as an oxidant for a variety of reactions crucial for metabolism. Central to this reaction are iron-containing enzymes which can react with oxygen to form high-spin iron(IV)-oxo transient intermediate. Although chemists have long sought to mimic this reactivity, the enzyme-like activation of oxygen to form high-spin iron(IV)-oxo species remains an unrealized goal. Here, we report a metal–organic framework, a porous material composed of metal ions connected through organic bridges to form 3-dimensional network, featuring iron(II) sites similar to that in α-ketoglutarate-dependent dioxygenase enzymes. The framework reacts with oxygen at low temperatures to form high-spin iron(IV)-oxo species and can oxidize cyclohexane and ethane. This result should inspire future catalyst development using environmentally friendly oxidants.
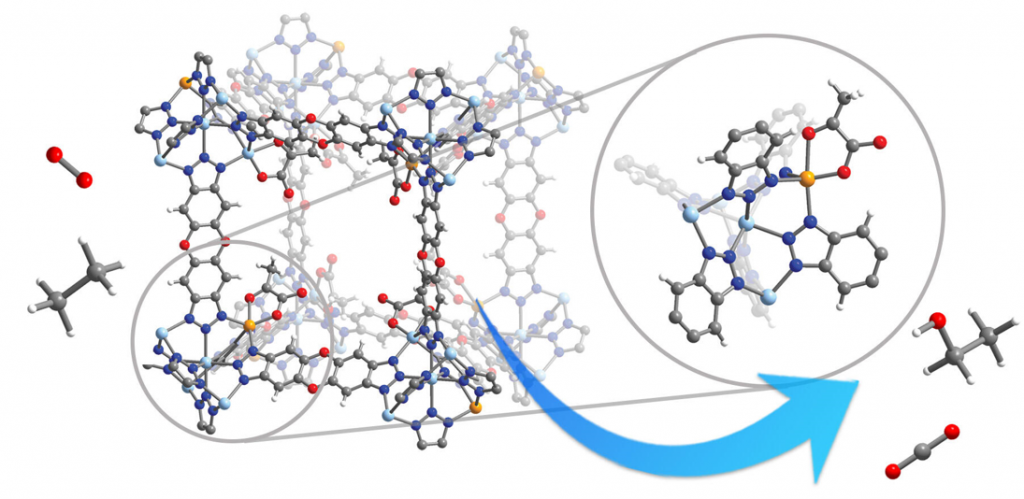
Keywords: iron oxo, metal–organic framework, oxygen activation, dioxygenase
Reference: Science 2023, 382, 547-553. DOI: 10.1126/science.add7417
