Sasirome Racochote, Phiphob Naweephattana, Panida Surawatanawong, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul and Darunee Soorukram*
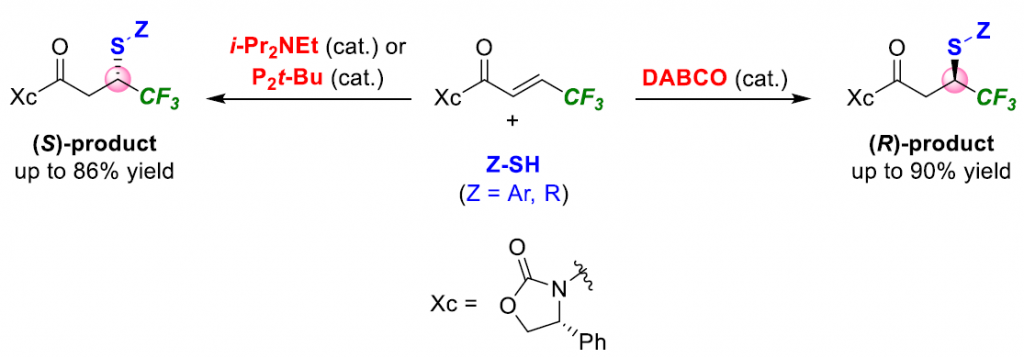
In conclusion, we reported the base-catalyzed diastereodivergent TMA of thiol derivatives, including thiophenols, alkyl, and benzyl thiols, to (R,E)-4-phenyl-3-(4,4,4- trifluorobut-2-enoyl)oxazolidin-2-one. A series of (R,S)- or (R,R)-TMA adducts was synthesized in moderate to good yields with high diastereoselectivities by simply changing the base-catalyst, including i-Pr2NEt, DABCO, or P2-t-Bu. A single diastereomer of each Michael adduct could be readily obtained by the usual column chromatography. The scale-up synthesis and the synthetic application to synthesize both enantiomers of biologically active chiral 2-(trifluoromethyl)thiochroman-4-ones and γ-trifluoromethyl γ-sulfone hydroxamates were demonstrated. The present work is complementary to the existing methods offering the advantages of, e.g., a switchable diastereoselectivity using a readily synthesized chiral starting material, a cheap and readily available base-catalyst, a simple and practical operation, and the involvement of a gram-scale synthesis, which enable synthetic applications in organic synthesis and related research fields.
Funding Information: This project is funded by the National Research Council of Thailand (NRCT) and Mahidol University: N42A650346. The authors thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation, and the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative. A student scholarship to S. R. from the Science Achievement Scholarship of Thailand (SAST) is gratefully acknowledged.
Reference : Sasirome Racochote, Phiphob Naweephattana, Panida Surawatanawong, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul and Darunee Soorukram.* Base-catalyzed diastereodivergent thia-Michael addition to chiral β-trifluoromethyl-α,β-unsaturated N-acylated oxazolidin-2-ones. Org. Biomol. Chem., 2023, 21, 7180.
