Bunyarat Rungtaweevoranit, Ali M. Abdel-Mageed, Pongtanawat Khemthong, Srisin Eaimsumang, Khetpakorn Chakarawet, Teera Butburee, Benny Kunkel, Sebastian Wohlrab, Kittipong Chainok, Jakkapop Phanthasri, Suttipong Wannapaiboon, Saran Youngjan, Theerada Seehamongkol, Sarawoot Impeng, Kajornsak Faungnawakij
Methane is the major component of natural gas and is an economically attractive fuel due to its abundance. However, being a gas, methane poses technical challenges in storage, and methane emitted into the atmosphere acts as a greenhouse gas that contributes to the climate change. Oxidation of methane to methanol, a high-valued product, thus is an economically and environmentally important process, of which a suitable catalyst is needed. Most oxidation catalysts face the problems of over-oxidation, in which the methanol produced is further oxidized to carbon dioxide. This work reports a novel catalyst based on a tunable solid called metal–organic framework doped with iron that oxidizes methane to methanol at high selectivity, fast rate, and is robust over a long period of time. A suite of spectroscopic methods identifies the iron ions isolated within the metal–organic framework as the active site where the reaction occurs.
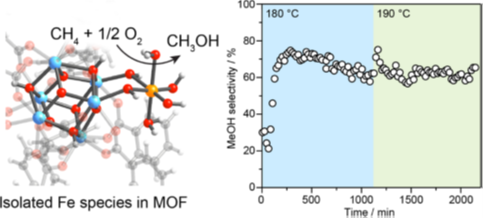
Keywords: heterogeneous catalysis, iron catalyst, metal−organic framework, methanol, partial methane oxidation
Reference: ACS Appl. Mater. Interfaces 2023, 15, 26700-26709. DOI: 10.1021/acsami.3c03310
