Thanapat Worakul†, and Panida Surawatanawong*,†,‡
†Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
‡Center of Sustainable Energy and Green Materials, Mahidol University, Salaya, Nakhon Pathom 73170, Thailand
*E-mail :panida.sur@mahidol.ac.th
The C–F bond transformation has attracted much attention in chemical synthesis. For aliphatic C(sp3)–F activation, strong main-group electrophiles are commonly employed to abstract the fluoride. Herein, we performed density functional calculations to gain insights into the hydrodefluorination of CF3-substituted aniline derivatives, CF3(C6H4)NMe2, by the Ru–S complex 1, [(PEt3)Ru(DmpS)]+ (DmpS = 2,6-dimesitylphenyl thiolate) with HSiMe2Ph. The mechanism involves three main steps: (i) the Si–H bond activation to generate Ru–H with thiosilane ligand, (ii) the fluoride abstraction by the sulfur-supported silylium (+SiMe2Ph) to generate +CF2(C6H4)NMe2, and (iii) the hydride transfer from the Ru-H to the carbocation to generate the first hydrodefluorination product, CF2H(C6H4)NMe2. To form the fully hydrodefluorinated product, the second and the third fluoride abstractions proceed with lower energy barriers than the first fluoride abstraction, which is the rate-determining step. As the Si-H activation generates Ru–H with thiosilane ligand, the Si-S bond must be cleaved to generate the active silylium moiety for the fluoride abstraction. In contrast, the Al-H activation of HAliBu2 leads to the formation of Ru–H with the alumenium (+AliBu2), stabilized by the donation from both (Ru–H) and sulfur lone pair. Upon fluoride abstraction, the donation from the sulfur lone pair to the alumenium remains while that from (Ru–H) is loosened and simply replaced by the donation from the fluoride. The fluoride abstraction by the alumenium has the higher interaction and also involves less structural change than that by the silylium. This corresponds with the higher activity for the C–F bond activation when HAliBu2 is used. The insights into the difference in the structures and activities in the fluoride abstraction by the silicon and the aluminum electrophiles, generated and stabilized by the Ru–S complex 1, will assist with the development of catalysts for C–F bond activation.
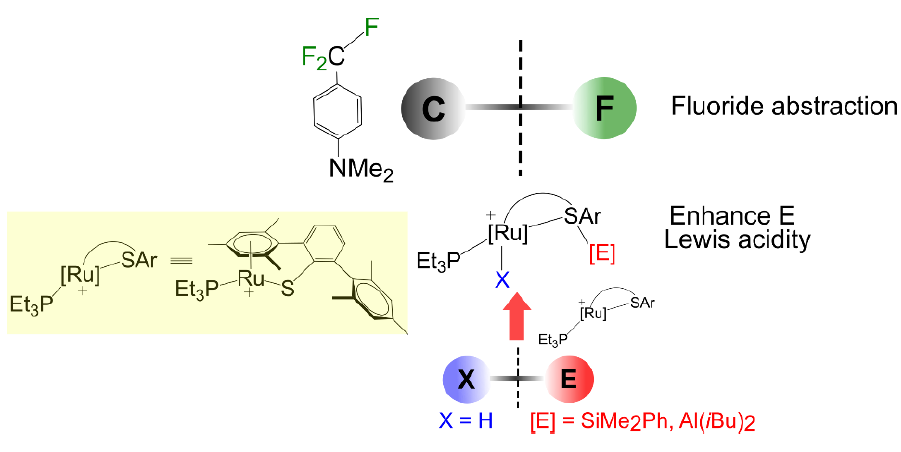
Reference: Worakul, T.; Surawatanawong, P.* Organometallics 2023, 42, 896. https://pubs.acs.org/doi/abs/10.1021/acs.organomet.3c00021
