Kritsadayut Lekjinda, Panya Sunintaboon *
Department of Chemistry, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
We report the green synthesis of trimethyl chitosan-functionalized poly(2-hydroxyethyl methacrylate) (PHEMATMC) nanogels via surfactant-free emulsion photopolymerization. TMC, a quaternized derivative of chitosan, was synthesized through methylation of chitosan, resulting in quaternary and tertiary amine groups as the main substitution products. TMC tertiary amine moiety and riboflavin (RF) acted as a redox photo-initiating system to generate free radicals for the polymerization under light irradiation. The effects of polymerization parameters such as irradiation time, concentrations of TMC and RF were investigated using MBA as crosslinker. Under the optimal condition of 1 % TMC, 4 % HEMA, 0.8 μM RF, 5 % MBA, and 4 h of polymerization time, the cationic PHEMA-TMC nanogel was synthesized with 76 % monomer conversion and an average diameter of about 106 nm. Moreover, the disulfide-crosslinked PHEMA-TMC nanogel was also synthesized using the disulfide dimethacrylate crosslinker, which exhibited a redox-induced degradation and release of encapsulated melatonin, potentially useful as a redox-responsive drug delivery carrier.
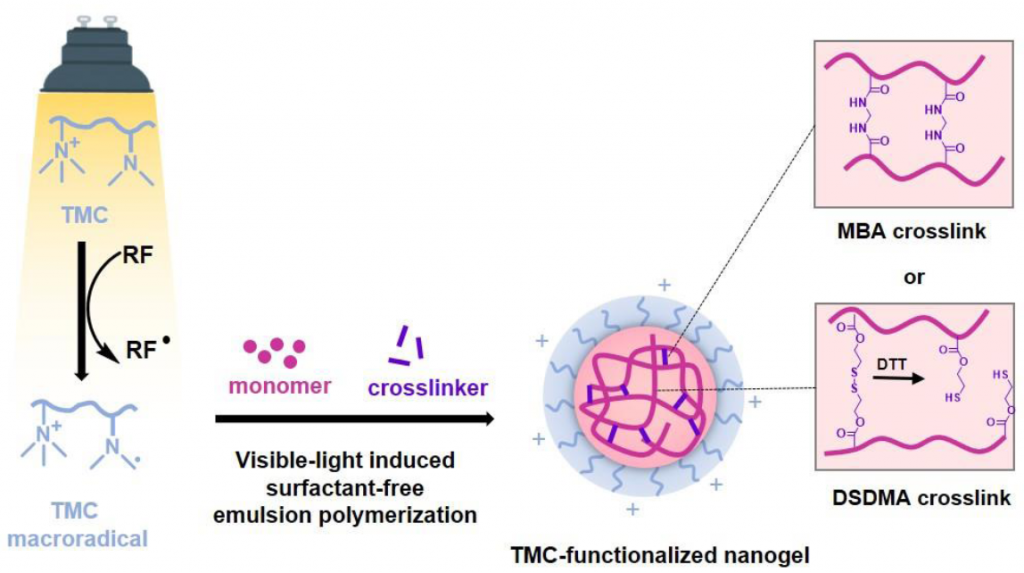
Keywords: Trimethyl chitosan; Riboflavin; PHEMA; Visible light-induced surfactant-free emulsion polymerization; Redox-responsive; Nanogel.
For more details: Carbohydrate Polymers Volume 304, 15 March 2023, 120495
https://doi.org/10.1016/j.carbpol.2022.120495
