Hathaichanok Sanjeam, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul, Darunee Soorukram*
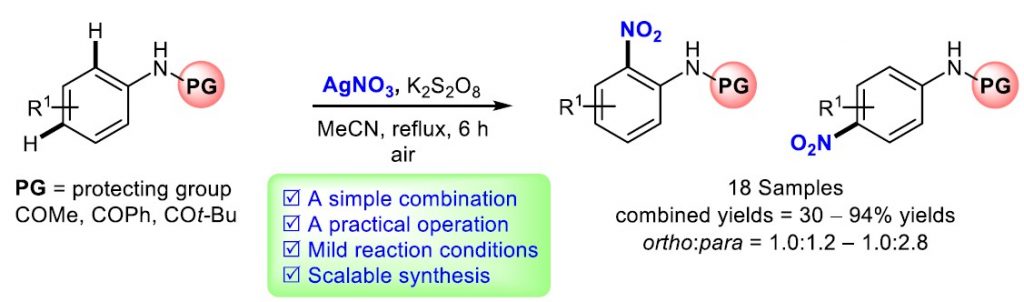
We reported nitration of N-acetyl anilides using a simple combination of AgNO3/K2S2O8. The advantages of the present procedure include the practical operation in an open-flask, considerably mild reaction conditions, and acceptable reaction time (6 h) without additional catalyst. Scale-up synthesis could also be performed in this study. Although both para- and ortho-nitroanilides were formed, they could be readily separable by using a simple chromatographic technique. Additionally, the synthetic entry to both para- and ortho-nitroanilides, especially those possessing interesting chemical and biological activities, should be attractive where the structure-activity relationship (SAR) study are required.
Funding Information: Thailand Research Fund (RSA6180025), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), and Ministry of Higher Education, Science, Research and Innovation, the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative. PERCH-CIC (student scholarship to H. S.).
Reference: Hathaichanok Sanjeam, Chutima Kuhakarn, Pawaret Leowanawat, Vichai
Reutrakul, Darunee Soorukram.* Nitration of N-acetyl anilides using silver(I)
nitrate/persulfate combination. Synthetic Communication. 2022, 52 (9–10), 1279–1289.
