Rungrawin Chatpreecha, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul, Darunee Soorukram*
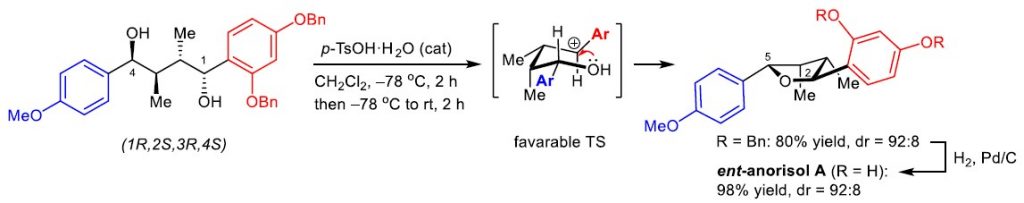
We have reported the asymmetric synthesis of three tetrahydrofuran (THF) lignans, namely (2S,3S,4R,5S)-, (2S,3S,4R,5R)-, and (2R,3S,4R,5R)-2-(2,4-dihydroxyphenyl)-5-(4-methoxyphenyl)-3,4-dimethyltetrahydrofurans, which are stereoisomers of natural anorisol A isolated from Anogeissus rivularis. The relative stereochemistry across the THF ring of each of the synthesized THF lignans was readily constructed with high yield and high stereoselectivity via an acid-catalyzed direct cyclization of the respective unprotected chiral 1,4-diarylbutane-1,4-diol bearing non-symmetrical aromatic rings. Comparison of the specific rotation values and the experimental ECD spectra of the three synthesized THF lignans with those of natural anorisol A confirms the 2R,3R,4S,5R configurations assigned for natural anorisol A. The structural and spectroscopic profiles of the synthesized stereoisomers of natural anorisol A would be of importance, especially for further development as potential chemotherapeutic agents. The developed asymmetric approach in this work highlights a practical and an atom-economic process that should also be applicable for further design of the asymmetric synthesis of other naturally occurring 2,5-diaryl-3,4-dimethyltetrahydrofurans.
Funding Information: National Research Council of Thailand (NRCT) and Mahidol University: N42A650346, the Center of Excellence for Innovation in Chemistry (PERCHCIC), Ministry of Higher Education, Science, Research and Innovation, and the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative. The Science Achievement Scholarship of Thailand (SAST) (student scholarship to R.C.).
Reference: Rungrawin Chatpreecha, Chutima Kuhakarn, Pawaret Leowanawat, Vichai
Reutrakul, Darunee Soorukram.* Asymmetric synthesis of ent-anorisol A and its
stereoisomers and confirmation of the absolute configuration of anorisol A isolated from
Anogeissus rivularis. Synthesis 2022, 54, 5324 – 5336.
