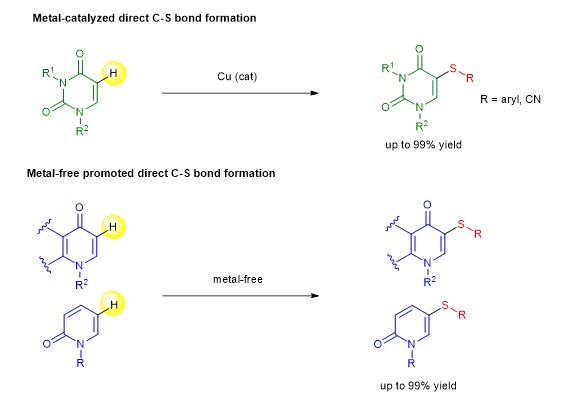
Aryl and heteroaryl sulfides are common structural motifs found in many bioactive natural products and drugs. These thio-substituted scaffolds are also important building blocks in synthesis, material science and pharmaceutical industry. Throughout the past two decades, several strategies to construct C−S bond; for instance, conventional synthesis, transition metal-catalyzed or metal-free mediated C−S bond cross coupling reactions using various forms of sulfur precursors under appropriate conditions, have been employed for the preparation of aryl sulfide-containing compounds. Among many methods, the development of a direct installation of the aryl sulfide moiety into non-prefunctionalized substrates via direct C−H bond functionalization has received significant attention from organic chemists in both academia and industry in recent years.
Our research developed a highly regioselective direct C–H thiolation (direct C–S bond formation via C−H bond functionalization strategy) of many types of bioactive N-heterocycles via metal-catalyzed and metal-free procedures. We found suitable reaction processes that enable the successful direct incorporation of a sulfide moiety into the uracil, quinolone and pyridone cores under regioselective and mild conditions, providing a useful and convenient approach for the preparation of a diverse array of thiosubstituted N-heterocycles in moderate to excellent yields.