Danupat Beukeaw[a] and Sirilata Yotphan[a]
*
[a] D. Beukeaw and Dr. S. Yotphan
Department of Chemistry and Center of Excellence for Innovation in Chemistry,
Faculty of Science, Mahidol University
Rama VI Road, Bangkok 10400, Thailand.
E-mail: sirilata.yot@mahidol.ac.th
https://chemistry.sc.mahidol.ac.th/en/people/faculty/sirilata-yotphan/
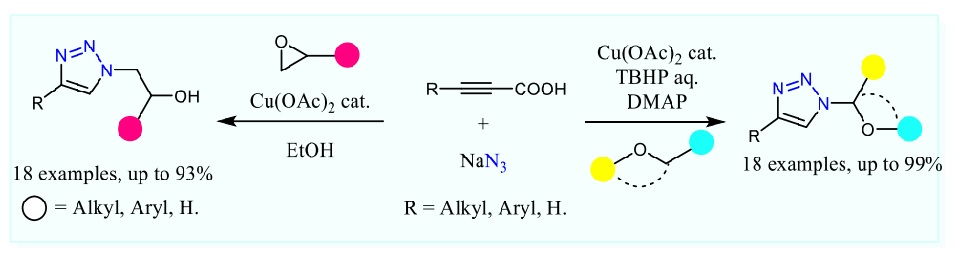
1,2,3-Triazoles are well-known five-membered N-heterocyclic motif, which have drawn significant interest due to their wide range of applications in synthesis, catalysis, coordination chemistry, pharmaceuticals, agrochemicals, polymers and materials chemistry. They are known to exhibit potent biological activities such as antiviral, anticancer, antifungal, antibacterial, anti-inflammatory, antimalarials and antioxidant activities. The common method for a preparation of the 1,2,3-triazole framework is the copper(I)-catalyzed 1,3-dipolar Huisgen cycloaddition reaction of azides with alkynes (CuAAC), which is the most remarkable example for “click” chemistry. Throughout the past decade, one-pot three-component methodologies for in situ generation of azides have been developed for the CuAAC to construct a variety of 1,4-disubstituted-1,2,3-triazole derivatives for further studies and utilization in many research fields. Herein, we wish to report a simple and convenient route for a one-pot preparation of 1,4-disub tituted-1,2,3-triazoles via Cu-catalyzed decarboxylative cycloaddition of alkynyl carboxylic acids with inexpensive and bench stable sodium azide and epoxides to give β-hydroxytriazoles in moderate to excellent yields. In addition, we found that the one-pot process for triazole synthesis using alkynyl carboxylic acids and sodium azide is compatible with ether substrates under the oxidation conditions. Both copper-catalyzed three-component reactions proceed very well under relatively benign and air-tolerant conditions, accommodate good substrate scope, give high yields and excellent regioselectivity of products in a single step operation, thus they can be used as an alternative synthetic protocol to prepare potent biologically tive analogues for further use in many applications.
Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation, Central Instrument Facility (CIF), Faculty
of Science, Mahidol University, and the Royal Golden Jubilee Ph.D. Program for supporting student scholarship to D.B. (Grant No.
PHD/0141/2560).
Reference: Copper-Catalyzed Decarboxylative Cycloaddition of Alkynyl Carboxylic Acids and Sodium Azide with Epoxides and
Ethers. Danupat Beukeaw and Sirilata Yotphan, ChemistrySelect 2021, 6, 9632 –9636.
doi.org/10.1002/slct.202102494
