Appended cation onto
an Fe complex directs oxidative selectivity
Teera Chantarojsiri
Electrostatic effects have been shown to affect efficiency and selectivity for different catalysts. In this work we reported an Fe NNN complex which can bind to cation, generating a local electric field. The redox potential of the Fe complex was tuned upon binding of cation with different charges (+1 vs. +2 vs. +3). Oxidation of C—H bond was also reported to be selective toward certain product upon binding of different cations.
This research project was supported by the Thailand Research Fund (grant no. MRG6280152) and Mahidol University (Basic Research Fund: fiscal year 2021). Single crystal X-ray crystallography were conducted in the collaboration with Assistant Professor Dr. Kittipong Chainok at Thammasat University Research Unit in Multifunctional Crystalline Materials and Applications (TU-MCMA), Faculty of Science and Technology, Thammasat University. Knowledge obtained from this work will benefit researchers who are studying electrostatic effects in chemical reactions, which will lead to the design of energy-efficient catalysts. Associated SDG goals are Affordable and Clean Energy (7) and Partnerships for the Goals (17).
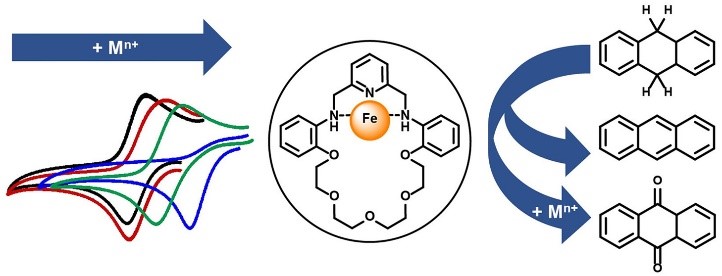
Reference: Incorporation of Cation Affects the Redox Reactivity of Fe–NNN Complexes on C–H Oxidation
Pathorn Teptarakulkarn, Wanutcha Lorpaiboon, Thana Anusanti, Natchapol Laowiwatkasem, Kittipong Chainok, Preeyanuch Sangtrirutnugul, Panida Surawatanawong, and Teera Chantarojsiri
Inorganic Chemistry Article ASAP DOI: 10.1021/acs.inorgchem.2c00762
