Asymmetric total synthesis of ventilanones A and B, two naturally occurring pyranonaphthoquinones from Ventilago harmandiana
Pramchai Deelertpaiboon, Sopanat Kongsriprapan, Suppachai Krajangsri, Nolan M. Betterley, Chutima Kuhakarn and Vichai Reutrakul
The asymmetric synthesis of pyranonaphthoquinones, namely ventilanone A and ventilanone B isolated from Ventilago harmandiana, was accomplished in 14 and 8 steps, respectively, employing L-rhamnose and gallic acid as the starting materials. The crucial reactions are the utilization of a newly introduced reagent, PhSCF2H/SnCl4, for the formylation of sterically hindered aromatics containing an electron-withdrawing methyl ester, and the efficient Hauser annulation of phenylthiophthalides with optically active C-1 glycal derived from L-rhamnose. The synthetic approaches are also potentially amenable to the introduction of other functional group at the C-4 and C-6 carbons of the pyranonaphthoquinones.
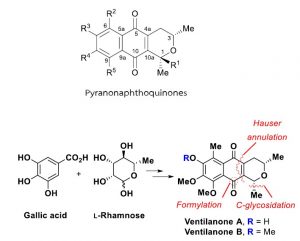
Asymmetric total synthesis of ventilanones A and B, two naturally occurring pyranonaphthoquinones
from Ventilago harmandiana. Synthesis, 2022, 54, 3093-3104.
