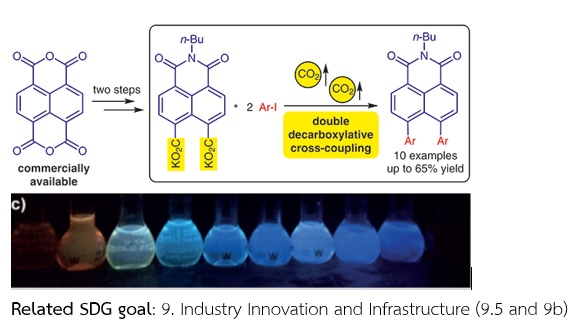
Research Title: Synthesis of peri-Diarylated Naphthalimides via Double Decarboxylative Cross-Coupling Reaction.
Institute: Department of Chemistry, Faculty of Science, Mahidol University.
Research Background and its significance: Naphthalimides represent one of the important classes of fluorophores for organic electronics. The introduction of various substituents at peri-positions results in a twisted conformation with tunable electronic properties. In this work, a convenient method based on palladium-catalyzed double decarboxylation cross-coupling for the preparation of peri-diarylated naphthalimides was developed. As a result, the absorption and emission properties of the naphthalimide derivatives could be readily modified for potential applications in organic optoelectronics.
Scope of Research: This work focused on the development of a facile synthesis of peri-diarylated naphthalimides via a palladium-catalyzed double decarboxylative cross-coupling reaction from commercially available naphthalenetetracarboxylic dianhydride (NTDA) and investigation of their optoelectronic properties by UV-vis absorption and fluorescence spectroscopic techniques.
Objectives: 1. To synthesize peri-diarylated naphthalimides via a palladium-catalyzed double decarboxylative cross-coupling reaction.
2. To study the substituent effect on photophysical properties of peri-diarylated naphthalimides.
Research grant: Thailand Research Fund (MRG6080128), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation, the Office of the Higher Education Commission and Faculty of Science, Mahidol University.
Collaboration: Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Mahidol University
Stakeholders: Scientific Community
Level of Collaboration: –
Output/outcome: New knowledge (Synthetic Methodology) and a publication.
Web link: doi: 10.1055/s-0039-1691062
Graphical abstract: