Sirisuk Keereewan, Chutima Kuhakarn, Pawaret Leowanawat, Saowanit Saithong, Vichai Reutrakul, Darunee Soorukram*
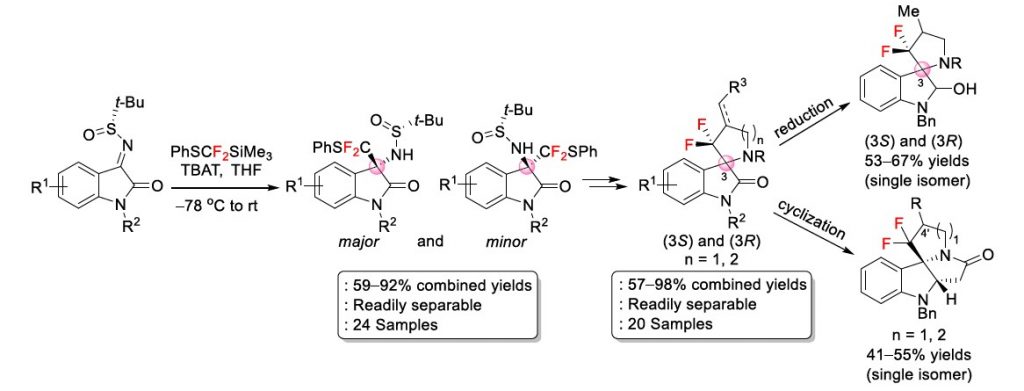
We successfully developed an efficient synthetic strategy to prepare enantioenriched gem-difluoromethylenated spiro-pyrrolidinyl and spiro-piperidinyl oxindoles. The reaction involved fluoride-mediated diastereoselective nucleophilic addition of PhSCF2SiMe3 to chiral N-tert-butanesulfinyl ketimines derived from isatins to install the quaternary chiral center at C-3 of the oxindole frameworks. The resulting adducts were further manipulated through the hydrolysis of the N-sulfinylimine and N-alkylation followed by reductive cleavage of the phenylsulfanyl group and radical cyclization leading to a series of chiral gem-difluoromethylenated spiro-pyrrolidinyl and spiropiperidinyl oxindoles, each as a single diastereomer after chromatographic purification. Synthetic transformations of the enantioenriched gem-difluoromethylenated spiropyrrolidinyl and spiro-piperidinyl oxindoles via reduction of the carbonyl moiety to give alcohol products as well as cyclization to give diaza-tetracyclic skeletons demonstrated the synthetic utility of the synthesized compounds of the present work which could have particular interest and significance in organic synthesis and pharmaceutical applications.
Funding Information: Mahidol University (NDFR 18/2565), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), the Ministry of Higher Education, Science, Research and Innovation, and the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative. The Science Achievement Scholarship of Thailand (SAST) (student scholarship to S.K.).
Reference: Sirisuk Keereewan, Chutima Kuhakarn, Pawaret Leowanawat, Saowanit
Saithong, Vichai Reutrakul, and Darunee Soorukram.* Diastereoselective Addition of
PhSCF2SiMe3 to Chiral N-tert-Butanesulfinyl Ketimines Derived from Isatins: Synthesis of
Enantioenriched gem-Difluoromethylenated Spiro-pyrrolidinyl and Spiro-piperidinyl
Oxindoles. J. Org. Chem. 2022, 87, 15963−15985.
