Kamonpan Sanachai, Panupong Mahalapbutr, Lueacha Tabtimmai, Supaphorn Seetaha,Tanakorn Kittikool, Sirilata Yotphan, Kiattawee Choowongkomon,* and Thanyada Rungrotmongkol*
Janus kinases (JAKs) are involved in a wide variety of cell signaling
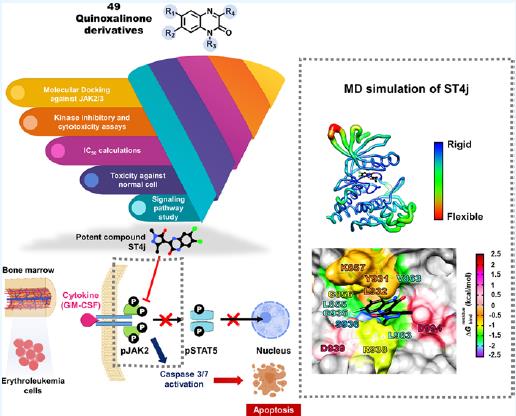
associated with T–cell and B–cell mediated diseases. The pathogenesis of common lymphoid–derived diseases and leukemia cancer has been implicated in JAK2 and JAK3. Therefore, to decrease the risk of these diseases, targeting this pathway using JAK2/3 inhibitors could serve as a valuable research tool. Herein, we used a combination of the computational and biological approaches to identify the quinoxalinone–based dual inhibitors of JAK2/3. First, an in–house library of 49 quinoxalinones was screened by molecular docking. Then, the inhibitory activities of 17 screened compounds against both JAKs as well as against two human erythroleukemia cell lines, TF1 and HEL were examined. The obtained results revealed that several quinoxalinones could potentially inhibit JAK2/3, and among them, ST4j showed strong inhibition against JAKs with the IC50 values of 13.00 ± 1.31 nM for JAK2 and 14.86 ± 1.29 nM for JAK3, which are better than ruxolitinib and tofacitinib. In addition, ST4j potentially inhibited TF1 cells (IC50 of 15.53 ± 0.82 μM) and HEL cells (IC50 of 17.90 ± 1.36 μM), similar to both tofacitinib ruxolitinib. Mechanistically, ST4j inhibited JAK2 autophosphorylation and induced cell apoptosis in dose– and time–dependent manners. From molecular dynamics simulations, ST4j was mainly stabilized by van der Waals interactions, and its hydroxyl group could form hydrogen bonds in the hinge region at residues S936 and R938 of JAK2. This research highlights the potential of ST4j to be a novel therapeutic agent for the treatment of lymphoid–derived diseases and leukemia cancer.