Concise synthesis and confirmation of the absolute configurationsof naturally occurring bioactive 2,7’-cyclolignans
Nannaphat Chumsri, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul, Darunee Soorukram*
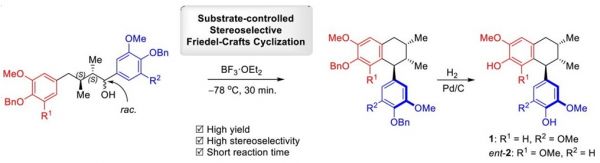
A concise asymmetric synthesis of naturally occurring bioactive 2,7’-cyclolignans, namely 4,4’-dihydroxy-3’,5,5’-trimethoxy-2,7’-cyclolignan and 4,4’-dihydroxy-3,3’,5-trimethoxy-2,7’-cyclolignan, possessing the uncommon 8,8’-syn dimethyl stereochemistry and unsymmetrical C-6 units with 7’,8’ -antiorientation is described using a substrate-controlled stereoselective Friedel-Crafts cyclization as the key step. The products were obtained in good yields with high stereoselectivity. The absolute configurations of natural 4,4’-dihydroxy-3’,5,5’-trimethoxy-2,7’-cyclolignan and those of natural 4,4’-dihydroxy3,3’,5-trimethoxy-2,7’-cyclolignan were assigned as (7’S,8S,8’S) and (7’R,8R,8’R), respectively, based on the experimental circular dichroism (CD) spectra of the corresponding synthesized compounds.
Reference: Concise synthesis and confirmation of the absolute configurationsof naturally occurring bioactive 2,7’-cyclolignans. Nannaphat Chumsri, Chutima Kuhakarn, Pawaret Leowanawat, Vichai Reutrakul, Darunee Soorukram*. Tetrahedron Lett. 2021, 66, 152827. https://doi.org/10.1016/j.tetlet.2021.152827
