Watchara Wimonsong and Sirilata Yotphan*
Department of Chemistry and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Faculty
of Science, Mahidol University, Rama VI Road, Bangkok 10400, Thailand.
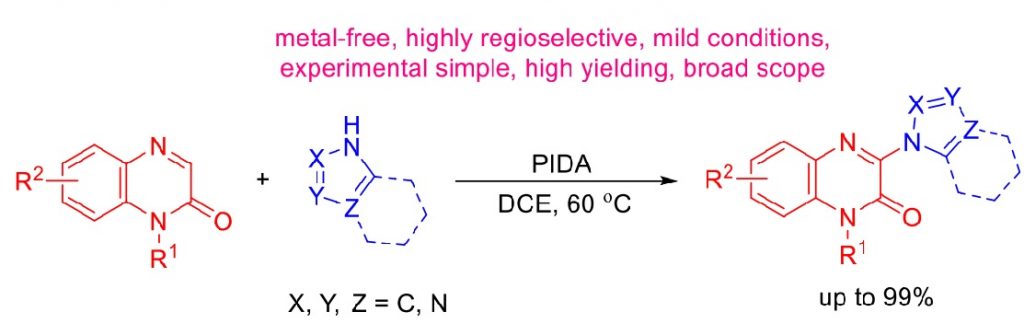
Herein, we report the PIDA-promoted direct C‒N bond coupling of quinoxalinones at C3 with N-heterocycles as nitrogen sources. This protocol features simple and mild metal-free conditions, high atom economy, employing readily available reagent, easy-to-handle experimental procedure, good to excellent product yields, together with broad substrate scope and good scalability. The present methodology provides a highly attractive and alternative approach to a preparation of a diverse range of 3-(azol-1-yl)quinoxalin-2(1H)-ones, which could be further employed in many applications. Acknowledgement: This work was supported by Thailand Research Fund (RSA6280024), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation, Central Instrument Facility (CIF), Faculty of Science, Mahidol University for providing research facilities, and Development and Promotion of Science and Technology Talent Project (DPST), the Institute for Promotion of Teaching Science and Technology for financial support through student scholarship to W.W.
Reference: PIDA-induced oxidative C-N bond coupling of quinoxalinones and azoles. Watchara Wimonsong and Sirilata
Yotphan*. Tetrahedron 81 (2021) 131919.
https://doi.org/10.1016/j.tet.2020.131919.
