Halogenated compounds are one of the most common volatile organic compounds (VOCs). They are highly valued chemicals in industry, due to their stability and low reactivity. Halogenated VOCs are safe to store and can be easily transported; nonetheless, their stability also means that these compounds are quite inert and accumulate in the atmosphere. Several studies show the total phenomenological overall rate coefficient of the reaction rather than specifics to the product yield. Clarification and quantification of the reaction pathways are of value to have accurate detailed chemical pathways. Nonetheless, detailed reaction pathways and kinetic analysis of the trifluoroethene reaction with the OH radical are still limited. This research examines the intermediate and subsequent oxidation reaction paths of trifluoroethene as shown below.
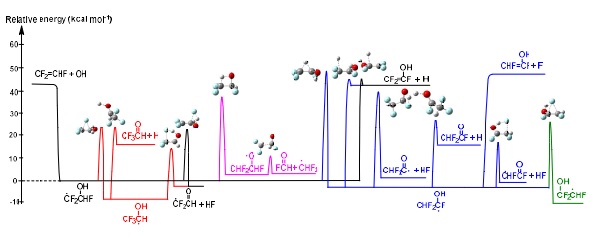
excellent agreement with experiment values. The study show that important forward
reactions result in: adduct stabilization, H atoms, hydrogen fluoride (HF) and
formation of fluorinated carbonyl radicals with CF2(=O) and CHF(=O) product
channels. Please visit reference for more details.
Reference: Suarwee Snitsiriwat, Suriyakit Yommee, and Joseph W. Bozzelli
The Journal of Physical Chemistry A 2021 125 (24), 5375-5384
DOI: 10.1021/acs.jpca.1c02390
