Preeyanuch Sangtrirutnugul
Ligands play a crucial role in stabilizing metal ions while influencing the overall structures, physical and magnetic properties of the metal complexes. In particular, judicious selection of ligands capable of bridging between two or more metal ions should allow some controls over the intramolecular metal–metal distances and spin–spin interactions, which directly affect various properties of the multinuclear metal complexes including magnetism and catalytic activity. This work investigates the synthesis and coordination chemistry of bis(triazolylmethyl)amine (BTA), which features two triazole rings connected by a secondary amine group, with Ni(II) ions. When one or two of the triazole rings of BTA feature pyridylmethyl substituent(s), the resulting dinuclear Ni(II) complexes are generated. On the other hand, when the triazolyl substituents are benzyl groups, the mononuclear Ni(II) product was obtained. Meanwhile, we also observe that the inorganic counterions, Cl– and NO3–, affect not only the structural arrangements of the corresponding dinuclear Ni(II) complexes but also their magnetic properties. This research project was funded by Thailand Research Fund (grant number: RSA6080082) and mainly conducted at Department of Chemistry, Faculty of Science, Mahidol University. Crystal data measurement and analysis were performed at VISTEC, Thailand, in collaboration with Assoc. Prof. Khamphee Phomphrai. Magnetic data were obtained at Osaka University with the help from Assoc. Prof. Nobuto Yoshinari and Prof. Takumi Konno. Insights into the formation of mono- and dinickel(II) complexes bridged by bis(triazolylmethyl)amine ligands and their resulting magnetic properties may be applicable to the area of molecular magnetism.
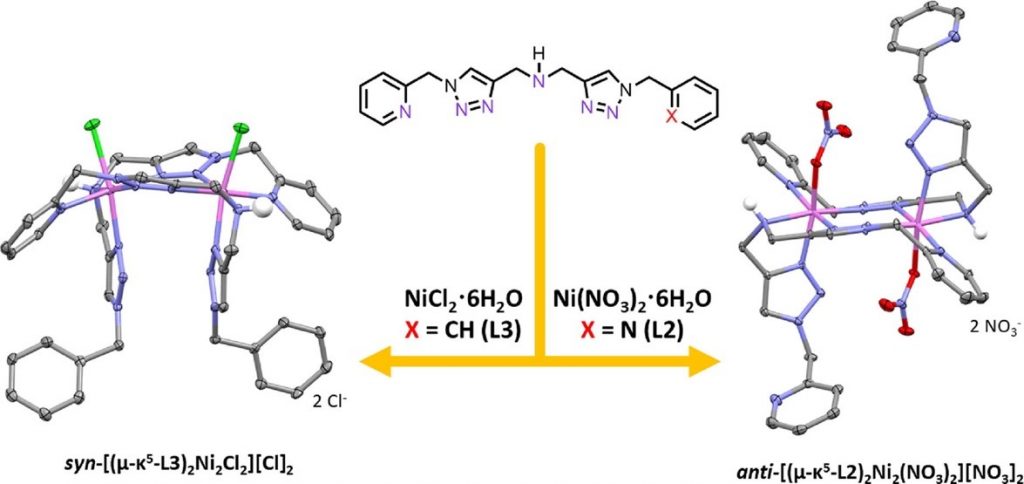
Reference: “Dinickel(II) complexes with pyridine-substituted bis(triazolylmethyl)amine ligands: structures and magnetic properties” Inthong, J.; Nakrajouyphon, V.; Udonsasporn, K.; Phomphrai, K.; Yoshinari, N.; Konno, T.; Sangtrirutnugul, P.* Polyhedron 2020, 191, 114813.
